Experimental Areas
The BIFTM programme aims to control cells in three different contexts:
A) Cells in the whole organism
The possibility to manipulate the behaviour of stem cells (e.g. neural stem cells, retinal stem cells, muscle stem cells (satellite cells) or cancer stem cells) in vivo in a highly specific manner would open the way for novel therapeutic approaches. For example, if we could manipulate stem cells in an embryo, one could foresee new therapeutic approaches, such as the correction of genetic defects.
Our aim is to generate knowledge, using various animal models, about how cell behaviour is naturally controlled and then to use this knowledge for the rational design of novel smart tools to control cells in vivo. Our starting points are the embryonic development of the eye and the central nervous system, the process of metastasis in cancer, and the plasticity of skeletal muscle. One particular focus will be the control of stem and progenitor cells which is highly relevant in each of our experimental model systems.
 Fluorescent image of a transgenic embryo from a zebrafish insertion line. |
The building of sophisticated culture devices represents a powerful strategy to manipulate stem cells in ways that are problematic or even impossible in vivo. This approach will naturally lead to direct biotechnological and biomedical applications.
| For example, stem cells isolated from an easily accessible population in the body could be expanded and then differentiated in vitro to produce a particular specialised cell type. Our aim will be to systematically explore how biological and physical cues in the local environment of cells contribute to their behaviour. We will then use material science technology to mimic these natural cues in artificial cell culture environments. The ultimate goal will be to develop smart culture devices able to efficiently "guide" the behaviour of stem or progenitor cells in vitro. |
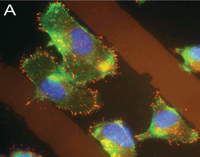 Fluorescent micrograph of cells growing on an artificially patterned surface. |
C) Bacterial biofilms on technical surfaces
Bacterial biofilms colonise natural and artificial surfaces, and thus represent a serious problem for industry and biomedicine. For example uncontrolled biofilm development can damage industrial surfaces, while in biomedical applications, the contamination of medical devices such as catheters by pathogenic bacteria poses a serious health threat. In contrast, in certain biotechnology applications biofilms composed of beneficial bacteria have to be established and maintained. Thus, it is vital to develop strategies, highly tailored for particular applications, to control biofilms.
Here the aim will be to systematically address how the properties of a surface contribute to the establishment and development of bacterial biofilms. We will then aim to construct "smart" surfaces, preferably with dynamic properties, that will allow us to harness the growth and composition of natural bacterial biofilms on artificial surfaces.
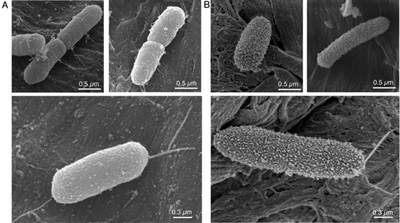 Electron micrograph of a biofilm forming bacterium (Pseudomonas aeruginosa) grown on a modified surface. |
